Magnetic Particle Imaging
Magnetic Particle Imaging (MPI) is an innovative medical imaging technology in which the spatial distribution of superparamagnetic iron oxide nanoparticles (SPIOs) is visualized with high-resolution and in real-time. The SPIOs are stimulated by a dynamic magnetic field and their response – which is proportional to the concentration of the SPIOs – is recorded. Spatial encoding of the particles is achieved by superimposing a magnetic gradient field that features a field free point (FFP) in its center. All particles at a distance from the FFP are saturated, thus, only the particles in the vicinity of the FFP contribute to the signal. MPI holds great promise as a tool in medical diagnostics and research due to its high sensitivity and specific contrast capabilities, allowing for precise representation of target structures. Additionally, the use of non-ionizing radiation makes this technique a safe alternative to conventional imaging methods such as positron emission tomography (PET) and computed tomography (CT).

Our group is one of a few sites worldwide that has access to a commercial preclinical MPI device which allows dynamic imaging with up to 46 volumes per second.
Projects
Multi-Frequency Magnetic Particle Imaging
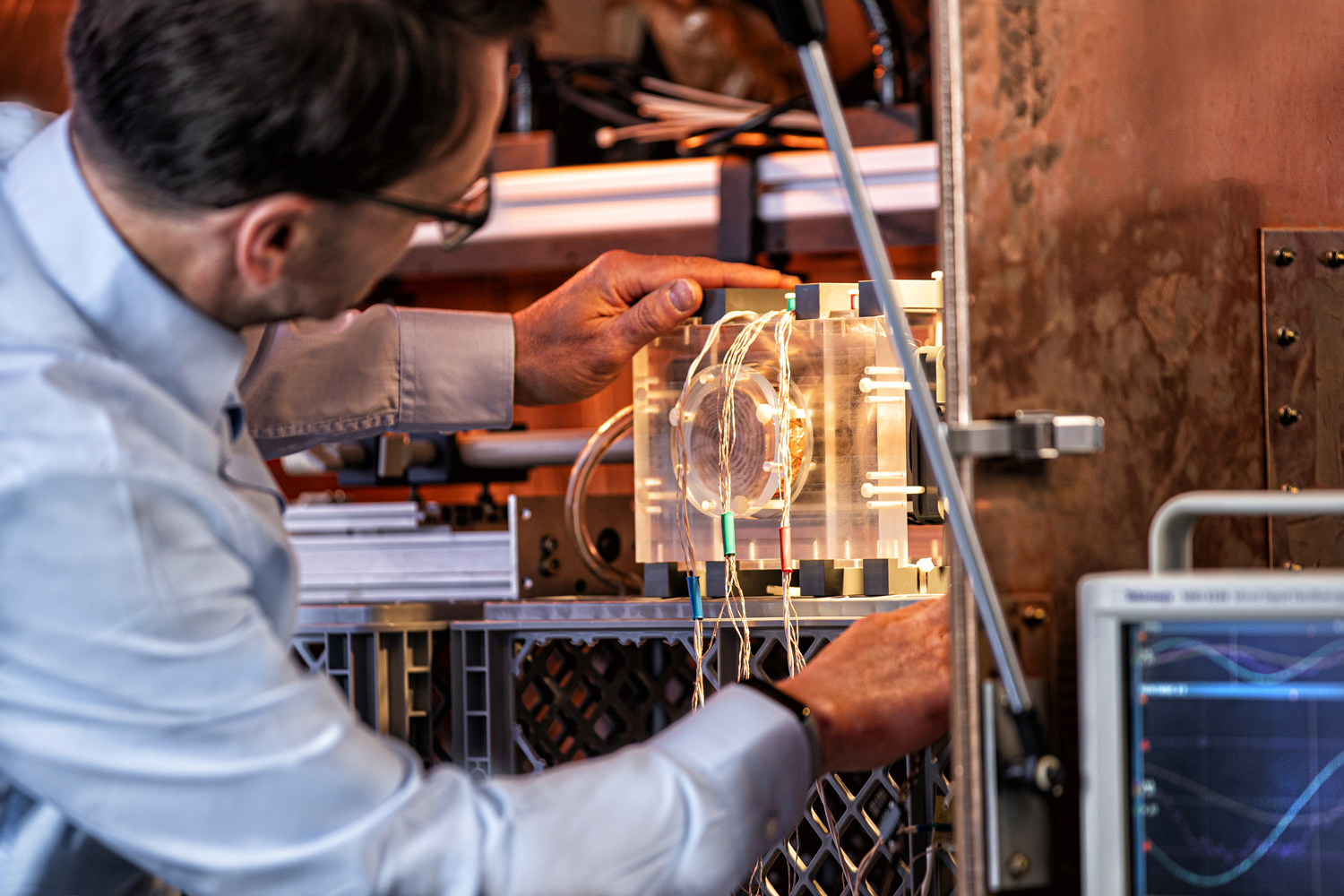
The project focuses on the simulation, design and evaluation of a novel in vivo imaging method for nanomedicine based on Magnetic Particle Imaging (MPI). The aim of this new method is to quantify the drug delivery of therapeutic agents in vivo for the field of nanotherapy, which would allow to predict and to optimize the success of nanotherapy in general. Currently, MPI is based on the spatially resolved measurement of the nonlinear distortion that occurs in the monofrequency excitation of superparamagnetic nanoparticles (SPIONs). Within this project, this approach will be extended by enabling multi-frequency excitation and thereby to be able to measure the relaxation properties (Neel and Brownian relaxation) of the SPIONs in vivo. This approach would allow us to distinguish circulating SPIONs from particles with restricted rotation, thus giving the option to quantify the Enhanced Permeability and Retention (EPR) effect as well as the uptake of the nanodrug into the cells in vivo. The project includes the design and evaluation of a novel field generator, the entire necessary software environment for generating and measuring different excitation waveforms, as well as the quantitative reconstruction of relaxation parameters from which further biologically relevant parameters (e.g. binding status and viscosity, pH value) and ambient temperature can be derived.
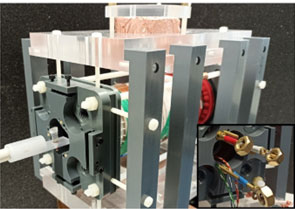
The prototype one-dimentional multi-frequency MPI scanner.
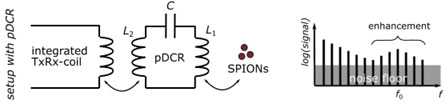
The passive dual coil resonator (pDCR)
Magnetic particle imaging (MPI) is a new tracer-based imaging technology that can determine the spatial distribution of superparamagnetic iron oxide-based nanoparticles (SPIONs) in vivo. SPIONs are single magnetic domain particles characterized by a nonlinear magnetization response to external magnetic field changes. MPI offers submillimeter spatial resolution and excellent temporal resolution at tens of volumes per second. While commercially available devices to date have been designed for preclinical and research purposes only, MPI has great potential to become a clinical tool due to its quantitative, depth-independent, non-invasive, and radiation-free nature. Further advances in sensitivity, spatial resolution, and tracer performance are needed for clinical implementation. Currently, the sensitivity and image resolution of preclinical MPI systems can be improved by using special receiving coils with separately tuned receiving electronics. The lowest reported iron detection limit of 2.8 μmol(Fe)/l was achieved with a highly sensitive gradiometric receive coil. However, this still exceeds the predicted iron detection limit of 0.1-1 μmol(Fe)/l by about an order of magnitude. In addition to hardware challenges (e.g., shielding, signal amplification, background drift) and the need for MPI-specific tracer optimization, a major reason for the observed deviation from the predicted detection limit is the systematic and stochastic noise in the measurement vector and calibration measurement. In this project proposal, we propose a cost-effective, handy, and purely passive coil insert that provides frequency-selective signal enhancement instead of using a dedicated receive coil with a separate and matched receive chain. The passive dual coil resonator is designed to fit within the bore of a preclinical MPI scanner. We hypothesize that the presented concept can be used to massively increase the sensitivity and spatial resolution of future MPI measurements via multi-resonance and multi-channel configurations. This DFG proposal aims to systematically investigate this hypothesis.